Impact of Processing and Physicochemical Parameter on Hibiscus sabdariffa Calyxes Biomolecules and Antioxidant Activity: From Powder Production to Reconstitution
Abstract
:1. Introduction
2. Hibiscus sabdariffa Plant
2.1. Production, Growing, and Culture
2.2. Chemical Composition
2.3. Food and Medicinal Uses
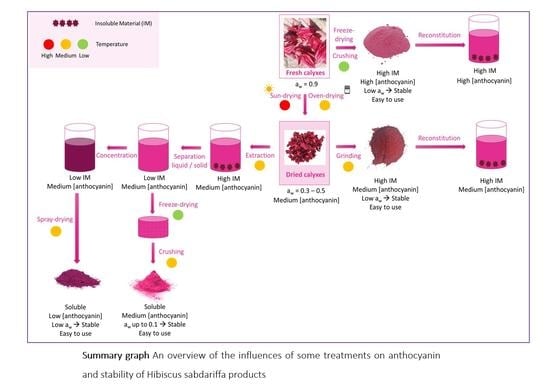
2.4. Interest in Stabilizing Hibiscus sabdariffa Calyx
- Alleviate the problem of the seasonality of hibiscus;
- Make the product available throughout the year;
- Ensure a long shelf life;
- Facilitate transport from a producing region to an importing region;
- Make the product accessible;
- Facilitate handling and use of the product;
- Facilitate the extraction of compounds of interest such as anthocyanin.
3. Stabilizing Processes and Impact on Products
3.1. Drying
3.1.1. Sun-Drying
3.1.2. Hot Air Drying
3.1.3. Dehumidified-Air-Drying
3.1.4. Microwave Drying
3.1.5. Infrared Drying
3.1.6. Impact of Drying Processes
3.2. Powder Production
3.2.1. Liquid Conversion into Solid Material
Spray-Drying
Freeze-Drying
3.2.2. Size Reduction by Dry Grinding
3.3. Powder Reconstitution and Biomolecule Extraction
3.3.1. Impact of Powder Physicochemical Properties on Reconstitution
3.3.2. Impact of Extrinsic Parameters on Reconstitution and Link with Extraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). Les Marchés Mondiaux Des Fruits et Légumes Biologiques. Available online: https://www.fao.org/3/y1669f/y1669f00.htm#Contents (accessed on 21 January 2022).
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef]
- Club DEMETER Pertes et Gaspillage Alimentaires. In Le Déméter 2022; Hors Collection; IRIS éditions, France; 2022; pp. 361–366. ISBN 0011662118. Available online: https://www.cairn.info/le-demeter-2022--0011662118.htm (accessed on 28 July 2023).
- Cisse, M. Couplage de Procédés Membranaires Pour la Production D’extraits Anthocyaniques: Application à l’Hibiscus sabdariffa; Montpellier SupAgro: Montpellier, France, 2010. [Google Scholar]
- Sinela, A.M. Etude des Mécanismes Réactionnels et des Cinétiques de Dégradation des Anthocyanes Dans un Extrait d’Hibiscus sabdariffa L.; Montpellier SupAgro: Montpellier, France, 2016. [Google Scholar]
- Navidad-Murrieta, M.S.; Pérez-Larios, A.; Sanchéz-Burgos, J.A.; Ragazzo-Sánchez, J.A.; Luna-Bárcenas, G.; Sáyago-Ayerdi, S.G. Use of a Taguchi Design in Hibiscus sabdariffa Extracts Encapsulated by Spray-Drying. Foods 2020, 9, 128. [Google Scholar] [CrossRef]
- Monteiro, M.J.P.; Costa, A.I.A.; Tomlins, K.I.; Pintado, M.E. Quality Improvement and New Product Development in the Hibiscus Beverage Industry. In Processing and Sustainability of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–183. ISBN 978-0-12-815259-1. [Google Scholar]
- Plotto, A.; Mazaud, F.; Röttger, A.; Steffel, K. HIBISCUS: Post-Production Management for Improved Market Access; FAO: Rome, Italy, 2004. [Google Scholar]
- Bagchi, D. Developing New Functional Food and Nutraceutical Products; Elsevier Science: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-802780-6. [Google Scholar]
- M’be, C.U.; Scher, J.; Petit, J.; Paris, C.; Amani, N.G.G.; Burgain, J. Effect of Powder Fractionation on Anthocyanin Extraction Kinetics during Powder Reconstitution. Powder Technol. 2023, 415, 118119. [Google Scholar] [CrossRef]
- Deli, M.; Petit, J.; Nguimbou, R.M.; Beaudelaire Djantou, E.; Njintang Yanou, N.; Scher, J. Effect of Sieved Fractionation on the Physical, Flow and Hydration Properties of Boscia senegalensis Lam., Dichostachys glomerata Forssk. and Hibiscus sabdariffa L. Powders. Food Sci. Biotechnol. 2019, 28, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Tham, T.C.; Ng, M.X.; Gan, S.H.; Chua, L.S.; Aziz, R.; Abdullah, L.C.; Ong, S.P.; Chin, N.L.; Law, C.L. Impacts of Different Drying Strategies on Drying Characteristics, the Retention of Bio-Active Ingredient and Colour Changes of Dried Roselle. Chin. J. Chem. Eng. 2018, 26, 303–316. [Google Scholar] [CrossRef]
- Juhari, N.H.; Martens, H.J.; Petersen, M.A. Changes in Physicochemical Properties and Volatile Compounds of Roselle (Hibiscus sabdariffa L.) Calyx during Different Drying Methods. Molecules 2021, 26, 6260. [Google Scholar] [CrossRef]
- Barani, Y.; Zhang, M.; Mujumdar, A.; Chang, L. Preservation of Color and Nutrients in Anthocyanin-rich Edible Flowers: Progress of New Extraction and Processing Techniques. J. Food Process. Preserv. 2022, 46, e16474. [Google Scholar] [CrossRef]
- Loyola Arenas, K.S.; Cruz, Y.; Victoria, M.T.; Vizcarra Mendoza, M.G.; Martínez Vera, C.; Anaya Sosa, I. Effect of Agitated Bed Drying on the Retention of Phenolic Compounds, Anthocyanins and Antioxidant Activity of Roselle (Hibiscus sabdariffa L.). Int. J. Food Sci. Technol. 2016, 51, 1457–1464. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A Phytochemical and Pharmacological Review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Ahmed, F.A.M.; Satti, N.M.E.; Eltahir, S.E.H. A Comparative Study on Some Major Constituents of Karkade (Hibiscus sabdariffa l.—Roselle Plant). Int. J. Pharma Biol. Sci. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Chumsri, P.; Sirichote, A.; Itharat, A. Studies on the Optimum Conditions for the Extraction and Concentration of Roselle (Hibiscus sabdariffa Linn.) Extract. Songklanakarin J. Sci. Technol. 2008, 30, 133–139. [Google Scholar]
- Ramírez-Rodrigues, M.M.; Balaban, M.O.; Marshall, M.R.; Rouseff, R.L. Hot and Cold Water Infusion Aroma Profiles of Hibiscus sabdariffa: Fresh Compared with Dried. J. Food Sci. 2011, 76, C212–C217. [Google Scholar] [CrossRef] [PubMed]
- Plotto, A.; Mazaud, F.; Röttger, A.; Steffel, K. HIBISCUS: Post-Harvest Operations; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2004. [Google Scholar]
- Gonzalez-Palomares, S.; Estarrón-Espinosa, M.; Gómez-Leyva, J.F.; Andrade-González, I. Effect of the Temperature on the Spray Drying of Roselle Extracts (Hibiscus sabdariffa L.). Plant Foods Hum. Nutr. 2009, 64, 62–67. [Google Scholar] [CrossRef]
- Abu-Tarboush, H.M.; Ahmed, S.A.B.; Kahtani, H.A.A. Some Nutritional and Functional Properties of Karkade (Hibiscus sabdariffa) Seed Products. Cereal Chem. 1997, 74, 352–355. [Google Scholar] [CrossRef]
- Ali, B.H.; Wabel, N.A.; Blunden, G. Phytochemical, Pharmacological and Toxicological Aspects of Hibiscus sabdariffa L.: A Review. Phytother. Res. 2005, 19, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Dafallah, A.A.; Al-Mustafa, Z. Investigation of the Anti-Inflammatory Activity of Acacia Nilotica and Hibiscus sabdariffa. Am. J. Chin. Med. 2012, 24, 263–269. [Google Scholar] [CrossRef]
- Wong, P.-K.; Yusof, S.; Ghazali, H.M.; Che Man, Y.B. Physico-Chemical Characteristics of Roselle (Hibiscus sabdariffa L.). Nutr. Food Sci. 2002, 32, 68–73. [Google Scholar] [CrossRef]
- Kabasakalis, V. Ascorbic Acid Content of Commercial Fruit Juices and Its Rate of Loss upon Storage. Food Chem. 2000, 70, 325–328. [Google Scholar] [CrossRef]
- Stinco, C.M.; Baroni, M.V.; Di Paola Naranjo, R.D.; Wunderlin, D.A.; Heredia, F.J.; Meléndez-Martínez, A.J.; Vicario, I.M. Hydrophilic Antioxidant Compounds in Orange Juice from Different Fruit Cultivars: Composition and Antioxidant Activity Evaluated by Chemical and Cellular Based (Saccharomyces cerevisiae) Assays. J. Food Compos. Anal. 2015, 37, 1–10. [Google Scholar] [CrossRef]
- Yaacob, M. Manual Teknologi Penanaman Rosel/Musa Yaacob, Engku Ismail Engku Ahmad, Yahaya Hussain; Institut Penyelidikan dan Pertanian Malaysia: Serdang, Malaysia, 2006; r2010; ISBN 978-967-936-506-1. [Google Scholar]
- Sánchez-Feria, C.; Salinas Moreno, Y.; Ybarra-Moncada, M.D.C.; González-Hernández, V.A.; Machuca-Sánchez, M.L. Effect of the Dehydration Method of Hibiscus sabdariffa L. Calyces on the Quality of Their Aqueous Extracts. Emir. J. Food Agric. 2021, 33, 159. [Google Scholar] [CrossRef]
- Jung, E.; Kim, Y.; Joo, N. Physicochemical Properties and Antimicrobial Activity of Roselle (Hibiscus sabdariffa L.). J. Sci. Food Agric. 2013, 93, 3769–3776. [Google Scholar] [CrossRef] [PubMed]
- Adewusi, S.R.A.; Ojumu, T.V.; Falade, O.S. The Effect of Processing on Total Organic Acids Content and Mineral Availability of Simulated Cassava-Vegetable Diets. Plant Foods Hum. Nutr. 1999, 53, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Chira, K.; Suh, J.-H.; Saucier, C.; Teissèdre, P.-L. Les polyphénols du raisin. Phytothérapie 2008, 6, 75–82. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Ruiz-Ramírez, A.; Baños, G.; El-Hafidi, M. Hibiscus sabdariffa Linnaeus (Malvaceae), Curcumin and Resveratrol as Alternative Medicinal Agents against Metabolic Syndrome. Cardiovasc. Hematol. Agents Med. Chem. 2013, 11, 25–37. [Google Scholar] [CrossRef]
- Tsai, P.-J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and Antioxidant Capacity in Roselle (Hibiscus sabdariffa L.) Extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Duangmal, K.; Saicheua, B.; Sueeprasan, S. Colour Evaluation of Freeze-Dried Roselle Extract as a Natural Food Colorant in a Model System of a Drink. LWT Food Sci. Technol. 2008, 41, 1437–1445. [Google Scholar] [CrossRef]
- D’Heureux-Calix, F.; Badrie, N. Consumer Acceptance and Physicochemical Quality of Processed Red Sorrel/Roselle (Hibiscus sabdariffa L.) Sauces from Enzymatic Extracted Calyces. Food Serv. Technol. 2004, 4, 141–148. [Google Scholar] [CrossRef]
- Johansen, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative Stress and the Use of Antioxidants in Diabetes: Linking Basic Science to Clinical Practice. Cardiovasc. Diabetol. 2005, 4, 5. [Google Scholar] [CrossRef]
- Lee, W.-C.; Wang, C.-J.; Chen, Y.-H.; Hsu, J.-D.; Cheng, S.-Y.; Chen, H.-C.; Lee, H.-J. Polyphenol Extracts from Hibiscus sabdariffa Linnaeus Attenuate Nephropathy in Experimental Type 1 Diabetes. J. Agric. Food Chem. 2009, 57, 2206–2210. [Google Scholar] [CrossRef]
- Fullerton, M.; Khatiwada, J.; Johnson, J.U.; Davis, S.; Williams, L.L. Determination of Antimicrobial Activity of Sorrel (Hibiscus sabdariffa) on Esherichia coli O157:H7 Isolated from Food, Veterinary, and Clinical Samples. J. Med. Food 2011, 14, 950–956. [Google Scholar] [CrossRef]
- Peng, C.-H.; Chyau, C.-C.; Chan, K.-C.; Chan, T.-H.; Wang, C.-J.; Huang, C.-N. Hibiscus sabdariffa Polyphenolic Extract Inhibits Hyperglycemia, Hyperlipidemia, and Glycation-Oxidative Stress While Improving Insulin Resistance. J. Agric. Food Chem. 2011, 59, 9901–9909. [Google Scholar] [CrossRef] [PubMed]
- Paim, M.P.; Maciel, M.J.; Weschenfelder, S.; Bergmann, G.P.; Avancini, C.A.M. Anti-Escherichia Coli Effect of Hibiscus sabdariffa L. in a Meat Model. Food Sci. Technol. 2017, 37, 647–650. [Google Scholar] [CrossRef]
- Seck, S.M.; Doupa, D.; Dia, D.G.; Diop, E.A.; Ardiet, D.-L.; Nogueira, R.C.; Graz, B.; Diouf, B. Clinical Efficacy of African Traditional Medicines in Hypertension: A Randomized Controlled Trial with Combretum micranthum and Hibiscus sabdariffa. J. Hum. Hypertens. 2018, 32, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aldapa, C.A.; Castro-Rosas, J.; Rangel-Vargas, E.; Navarro-Cortez, R.O.; Cabrera-Canales, Z.E.; Díaz-Batalla, L.; Martínez-Bustos, F.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. A Modified Achira (Canna indica L.) Starch as a Wall Material for the Encapsulation of Hibiscus sabdariffa Extract Using Spray Drying. Food Res. Int. 2019, 119, 547–553. [Google Scholar] [CrossRef]
- Sabarez, H.T. 4—Modelling of Drying Processes for Food Materials. In Modeling Food Processing Operations; Bakalis, S., Knoerzer, K., Fryer, P.J., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2015; pp. 95–127. ISBN 978-1-78242-284-6. [Google Scholar]
- Chen, X.D.; Mujumdar, A.S. Drying Technologies in Food Processing; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-1-4443-0942-3. [Google Scholar]
- Nguyen, Q.V.; Chuyen, H.V. Processing of Herbal Tea from Roselle (Hibiscus sabdariffa L.): Effects of Drying Temperature and Brewing Conditions on Total Soluble Solid, Phenolic Content, Antioxidant Capacity and Sensory Quality. Beverages 2020, 6, 2. [Google Scholar] [CrossRef]
- Marnoto, T. Drying of Rosella (Hibiscus sabdariffa) Flower Petals Using Solar Dryer with Double Glass Cover Collector. Int. J. Sci. Eng. 2014, 7, 150–154. [Google Scholar] [CrossRef]
- Sankalpa, K.B.; Ramachandra, C.T.; Dinesha, B.L.; Nidoni, U.K.; Hiregoudar, S.; Beladhadi, R.V. Effect of Different Drying and Grinding Methods on Biochemical Properties of Sweet Orange Peel Powder. Asian J. Dairy & Food Res. 2017, 36, 260–263. [Google Scholar] [CrossRef]
- Ledesma-Valladolid, J.P.; Reynoso-Camacho, R.; Nava-Morales, G.M.; Vázquez-Barrios, M.E.; Vázquez-Celestino, D.; Dufoo-Hurtado, M.D.; Mercado-Silva, E.M. Quality Properties of Roselle (Hibiscus sabdariffa) Calyxes as Affected by Drying Process. Acta Hortic. 2020, 1287, 145–152. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Mujumdar, A.S.; Mothibe, K.J. Microwave-Assisted Pulse-Spouted Bed Freeze-Drying of Stem Lettuce Slices—Effect on Product Quality. Food Bioprocess. Technol. 2013, 6, 3530–3543. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Mujumdar, A.S.; Liu, Y. Recent Developments in Key Processing Techniques for Oriental Spices/Herbs and Condiments: A Review. Food Rev. Int. 2022, 38, 1791–1811. [Google Scholar] [CrossRef]
- Shivanna, V.B.; Subban, N. Carotenoids Retention in Processed Curry Leaves (Murraya koenigii L. Spreng). Int. J. Food Sci. Nutr. 2013, 64, 58–62. [Google Scholar] [CrossRef]
- Pawar, S.B.; Pratape, V.M. Fundamentals of Infrared Heating and Its Application in Drying of Food Materials: A Review. J. Food Process Eng. 2017, 40, e12308. [Google Scholar] [CrossRef]
- Adak, N.; Heybeli, N.; Ertekin, C. Infrared Drying of Strawberry. Food Chem. 2017, 219, 109–116. [Google Scholar] [CrossRef]
- Jin, W.; Mujumdar, A.S.; Zhang, M.; Shi, W. Novel Drying Techniques for Spices and Herbs: A Review. Food Eng. Rev. 2018, 10, 34–45. [Google Scholar] [CrossRef]
- Joardder, M.U.H.; Kumar, C.; Karim, M.A. Food Structure: Its Formation and Relationships with Other Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1190–1205. [Google Scholar] [CrossRef]
- Koua, B.K.; Koffi, P.M.E.; Gbaha, P. Evolution of Shrinkage, Real Density, Porosity, Heat and Mass Transfer Coefficients during Indirect Solar Drying of Cocoa Beans. J. Saudi Soc. Agric. Sci. 2019, 18, 72–82. [Google Scholar] [CrossRef]
- Joardder, M.U.H.; Karim, A.; Kumar, C.; Brown, R.J. Relationship Between Drying Conditions, Pore Characteristics, and Food Quality. In Porosity: Establishing the Relationship between Drying Parameters and Dried Food Quality; Joardder, M.U.H., Karim, A., Kumar, C., Brown, R.J., Eds.; SpringerBriefs in Food, Health, and Nutrition; Springer International Publishing: Cham, Switzerland, 2016; pp. 65–68. ISBN 978-3-319-23045-0. [Google Scholar]
- Bonazzi, C.; Dumoulin, E. Quality Changes in Food Materials as Influenced by Drying Processes. In Modern Drying Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 1–20. ISBN 978-3-527-63166-7. [Google Scholar]
- Seerangurayar, T.; Al-Ismaili, A.M.; Jeewantha, L.H.J.; Al-Nabhani, A. Experimental Investigation of Shrinkage and Microstructural Properties of Date Fruits at Three Solar Drying Methods. Sol. Energy 2019, 180, 445–455. [Google Scholar] [CrossRef]
- Ramos, I.N.; Brandão, T.R.S.; Silva, C.L.M. Structural Changes During Air Drying of Fruits and Vegetables. Food Sci. Technol. Int. 2003, 9, 201–206. [Google Scholar] [CrossRef]
- Albanese, D.; Cinquanta, L.; Cuccurullo, G.; Di Matteo, M. Effects of Microwave and Hot-Air Drying Methods on Colour, β-Carotene and Radical Scavenging Activity of Apricots. Int. J. Food Sci. Technol. 2013, 48, 1327–1333. [Google Scholar] [CrossRef]
- Baysal, T.; Icier, F.; Ersus, S.; Yıldız, H. Effects of Microwave and Infrared Drying on the Quality of Carrot and Garlic. Eur. Food Res. Technol. 2003, 218, 68–73. [Google Scholar] [CrossRef]
- Jackman, R.L.; Yada, R.Y.; Tung, M.A.; Speers, R.A. Anthocyanins as Food Colorants—A Review. J. Food Biochem. 1987, 11, 201–247. [Google Scholar] [CrossRef]
- Alibas, I. Microwave, Vacuum, and Air Drying Characteristics of Collard Leaves. Dry. Technol. 2009, 27, 1266–1273. [Google Scholar] [CrossRef]
- Balzarini, M.F.; Reinheimer, M.A.; Ciappini, M.C.; Scenna, N.J. Comparative Study of Hot Air and Vacuum Drying on the Drying Kinetics and Physicochemical Properties of Chicory Roots. J. Food Sci. Technol. 2018, 55, 4067–4078. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhang, M.; Sun, J. Physico-Chemical Properties of Cabbage Powder as Affected by Drying Methods. Dry. Technol. 2007, 25, 913–916. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, Q.; Liu, J.; Zhao, C.; Xue, F.; Zhao, Y. Decomposition of Five Phenolic Compounds in High Temperature Water. J. Braz. Chem. Soc. 2014, 25, 2102–2107. [Google Scholar] [CrossRef]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Thermal Degradation of Acylated and Nonacylated Anthocyanins. J. Food Sci. 2006, 71, C504–C512. [Google Scholar] [CrossRef]
- Seeram, N.P.; Bourquin, L.D.; Nair, M.G. Degradation Products of Cyanidin Glycosides from Tart Cherries and Their Bioactivities. J. Agric. Food Chem. 2001, 49, 4924–4929. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of Convective and Vacuum–Microwave Drying on the Bioactive Compounds, Color, and Antioxidant Capacity of Sour Cherries. Food Bioprocess. Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Nyam, K.L. Effect of Different Drying Methods on the Physical Properties and Antioxidant Activities of Hibiscus Cannabinus Leaves. Food Meas. 2019, 13, 1279–1286. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, Y.; Zhou, W.; Zhu, D. Hot Air Drying of Sipunculus Nudus: Effect of Microwave-Assisted Drying on Quality and Aroma. Foods 2023, 12, 733. [Google Scholar] [CrossRef]
- Dehydration Processes and Their Influence on Powder Properties. In Analytical Methods for Food and Dairy Powders; Wiley-Blackwell: Oxford, UK, 2012; pp. 1–43. ISBN 978-1-118-30739-7.
- Djaeni, M.; Kumoro, A.C.; Sasongko, S.B.; Utari, F.D. Drying Rate and Product Quality Evaluation of Roselle (Hibiscus sabdariffa L.) Calyces Extract Dried with Foaming Agent under Different Temperatures. Int. J. Food Sci. 2018, 2018, 9243549. [Google Scholar] [CrossRef]
- Idham, Z.; Muhamad, I.I.; Sarmidi, M.R. Degradation Kinetics and Color Stability of Spray-Dried Encapsulated Anthocyanins from Hibiscus sabdariffa L.: Stability of Spray Dried Anthocyanins. J. Food Process Eng. 2012, 35, 522–542. [Google Scholar] [CrossRef]
- Beristain, C.J.; Vernon-Carter, E.I. Studies on the Interaction of Arabic (Acacia senegal) and Mesquite (Prosopis juliflora) Cum as Emulsion Stabilizing Agents for Spray-Dried Encapsulated Orange Peel Oil. Dry. Technol. 1995, 13, 455–461. [Google Scholar] [CrossRef]
- Ré, M.I. Microencapsulation by Spray Drying. Dry. Technol. 1998, 16, 1195–1236. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Maudhuit, A.; Gaiani, C.; Desobry, S. Encapsulation of Bioactive Compounds Using Competitive Emerging Techniques: Electrospraying, Nano Spray Drying, and Electrostatic Spray Drying. J. Food Eng. 2023, 339, 111260. [Google Scholar] [CrossRef]
- Moejes, S.N.; Visser, Q.; Bitter, J.H.; van Boxtel, A.J.B. Closed-Loop Spray Drying Solutions for Energy Efficient Powder Production. Innov. Food Sci. Emerg. Technol. 2018, 47, 24–37. [Google Scholar] [CrossRef]
- Eroğlu, E.; Tontul, İ.; Topuz, A. Optimization of Aqueous Extraction and Spray Drying Conditions for Efficient Processing of Hibiscus Blended Rosehip Tea Powder. J. Food Process Preserv. 2018, 42, e13643. [Google Scholar] [CrossRef]
- Largo Avila, E.; Cortes Rodríguez, M.; Ciro Velásquez, H.J. Influence of Maltodextrin and Spray Drying Process Conditions on Sugarcane Juice Powder Quality. Rev. Fac. Nac. De Agron. Medellín 2015, 68, 7509–7520. [Google Scholar] [CrossRef]
- Langrish, T.; Chiou, D. Producing Powders of Hibiscus Extract in a Laboratory-Scale Spray Dryer. Int. J. Food Eng. 2008, 4, 1411. [Google Scholar] [CrossRef]
- Cassol, L.; Noreña, C.P.Z. Microencapsulation and Accelerated Stability Testing of Bioactive Compounds of Hibiscus sabdariffa. Food Meas. 2021, 15, 1599–1610. [Google Scholar] [CrossRef]
- Nur Fitriani, U.; Yusuf, M.; Ilyas, F.S. Spray Drying of Rosella (Hibiscus sabdariffa L.) Powder: Effect of Shelf Life on Physicochemical Properties and Cyanidin 3-O—Glucoside. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012002. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of Spray Drying, Freeze Drying and Convective Hot Air Drying for the Production of a Probiotic Orange Powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Díaz-Bandera, D.; Villanueva-Carvajal, A.; Dublán-García, O.; Quintero-Salazar, B.; Dominguez-Lopez, A. Assessing Release Kinetics and Dissolution of Spray-Dried Roselle (Hibiscus sabdariffa L.) Extract Encapsulated with Different Carrier Agents. LWT Food Sci. Technol. 2015, 64, 693–698. [Google Scholar] [CrossRef]
- Fellows, P. Food Processing Technology: Principles and Practice, 4th ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Amsterdam, The Netherlands; Cambridge, MA, USA, 2017; ISBN 978-0-08-101907-8. [Google Scholar]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of Grape (Vitis labrusca var. Bordo) Skin Phenolic Extract Using Gum Arabic, Polydextrose, and Partially Hydrolyzed Guar Gum as Encapsulating Agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Mozetič, B.; Trebše, P.; Simčič, M.; Hribar, J. Changes of Anthocyanins and Hydroxycinnamic Acids Affecting the Skin Colour during Maturation of Sweet Cherries (Prunus avium L.). LWT Food Sci. Technol. 2004, 37, 123–128. [Google Scholar] [CrossRef]
- Brønnum-Hansen, K.; Jacobsen, F.; Flink, J.M. Anthocyanin Colourants from Elderberry (Sambucus nigra L.). 1. Process Considerations for Production of the Liquid Extract. Int. J. Food Sci. Technol. 1985, 20, 703–711. [Google Scholar] [CrossRef]
- Thakur, B.R.; Arya, S.S. Studies on Stability of Blue Grape Anthocyanins. Int. J. Food Sci. Technol. 1989, 24, 321–326. [Google Scholar] [CrossRef]
- Gradinaru, G.; Biliaderis, C.G.; Kallithraka, S.; Kefalas, P.; Garcia-Viguera, C. Thermal Stability of Hibiscus sabdariffa L. Anthocyanins in Solution and in Solid State: Effects of Copigmentation and Glass Transition. Food Chem. 2003, 83, 423–436. [Google Scholar] [CrossRef]
- Brouillard, R.; Mazza, G.; Saad, Z.; Albrecht-Gary, A.M.; Cheminat, A. The Co-Pigmentation Reaction of Anthocyanins: A Microprobe for the Structural Study of Aqueous Solutions. J. Am. Chem. Soc. 1989, 111, 2604–2610. [Google Scholar] [CrossRef]
- Chandra, A.; Nair, M.G.; Iezzoni, A.F. Isolation and Stabilization of Anthocyanins from Tart Cherries (Prunus cerasus L.). J. Agric. Food Chem. 1993, 41, 1062–1065. [Google Scholar] [CrossRef]
- Chamayou, A.; Fages, J. Broyage dans les industries agroalimentaires. In Technologie des Pulvérulents Dans Les IAA; Ilari, J.-L., Melcion, J.-P., Eds.; Sciences & Techniques Agroalimentaires; Lavoisier: Cachan, France, 2003; chapter 13; pp. 375–406. [Google Scholar]
- Hulin, J.P. Hydrodynamics of Dispersed Media: Articles Based on Presentations Made at the 4th EPS Liquid State Conference on the Hydrodynamics of Dispersed Media Held in Arcachon, France, 24–27 May 1988; Random Materials and Processes; North-Holland; Sole Distributors for the U.S.A. and Canada, Elsevier Science Pub. Co.: Amsterdam, The Netherlands; New York, NY, USA, 1990; Volume 1, ISBN 978-0-444-88356-8. [Google Scholar]
- Baggenstoss, J.; Perren, R.; Escher, F. Water Content of Roasted Coffee: Impact on Grinding Behaviour, Extraction, and Aroma Retention. Eur. Food Res. Technol. 2008, 227, 1357–1365. [Google Scholar] [CrossRef]
- Becker, L.; Zaiter, A.; Petit, J.; Zimmer, D.; Karam, M.-C.; Baudelaire, E.; Scher, J.; Dicko, A. Improvement of Antioxidant Activity and Polyphenol Content of Hypericum perforatum and Achillea millefolium Powders Using Successive Grinding and Sieving. Ind. Crops Prod. 2016, 87, 116–123. [Google Scholar] [CrossRef]
- Zaiter, A.; Becker, L.; Petit, J.; Zimmer, D.; Karam, M.-C.; Baudelaire, É.; Scher, J.; Dicko, A. Antioxidant and Antiacetylcholinesterase Activities of Different Granulometric Classes of Salix alba (L.) Bark Powders. Powder Technol. 2016, 301, 649–656. [Google Scholar] [CrossRef]
- M’be, C.U.; Scher, J.; Petit, J.; Amani, N.G.; Burgain, J. Relationship between Drying and Grinding Parameters and Physicochemical Properties of Hibiscus sabdariffa Calyx Powders. Part. Sci. Technol. 2023, 41, 32–41. [Google Scholar] [CrossRef]
- Ghodki, B.M.; Goswami, T.K. Effect of Grinding Temperatures on Particle and Physicochemical Characteristics of Black Pepper Powder. Powder Technol. 2016, 299, 168–177. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire Djantou, E.; Scher, J. Effects of Drying and Grinding in Production of Fruit and Vegetable Powders: A Review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Singh, S.S.; Ghodki, B.M.; Goswami, T.K. Effect of Grinding Methods on Powder Quality of King Chilli. Food Meas. 2018, 12, 1686–1694. [Google Scholar] [CrossRef]
- Kim, A.-N.; Kim, H.-J.; Kerr, W.L.; Choi, S.-G. The Effect of Grinding at Various Vacuum Levels on the Color, Phenolics, and Antioxidant Properties of Apple. Food Chem. 2017, 216, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Builders, P.F.; Ezeobi, C.R.; Tarfa, F.D.; Builders, M.I. Assessment of the Intrinsic and Stability Properties of the Freeze-Dried and Formulated Extract of Hibiscus sabdariffa Linn. Malvaceae 2010, 4, 304–310. [Google Scholar]
- Cid-Ortega, S.; Guerrero-Beltran, J.A. Roselle Calyces Particle Size Effect on the Physicochemical and Phytochemicals Characteristics. J. Food Res. 2014, 3, 83. [Google Scholar] [CrossRef]
- Tan, S.L.; Sulaiman, R. Color and Rehydration Characteristics of Natural Red Colorant of Foam Mat Dried Hibiscus sabdariffa L. Powder. Int. J. Fruit. Sci. 2020, 20, 89–105. [Google Scholar] [CrossRef]
- Petit, J.; Burgain, J.; Gaiani, C.; Scher, J. Aptitude à L’écoulement de Poudres Alimentaires: Impact Des Propriétés Physicochimiques Des Particules; Industries Alimentaires et Agricoles (IAA): Paris, France, 2017; pp. 26–30. [Google Scholar]
- Fitzpatrick, J.J.; Hodnett, M.; Twomey, M.; Cerqueira, P.S.M.; O’Flynn, J.; Roos, Y.H. Glass Transition and the Flowability and Caking of Powders Containing Amorphous Lactose. Powder Technol. 2007, 178, 119–128. [Google Scholar] [CrossRef]
- Gnagne, E.H.; Petit, J.; Gaiani, C.; Scher, J.; Amani, G.N. Characterisation of Flow Properties of Foutou and Foufou Flours, Staple Foods in West Africa, Using the FT4 Powder Rheometer. Food Meas. 2017, 11, 1128–1136. [Google Scholar] [CrossRef]
- Petit, J.; Michaux, F.; Jacquot, C.; Chávez Montes, E.; Dupas, J.; Girard, V.; Gianfrancesco, A.; Scher, J.; Gaiani, C. Storage-Induced Caking of Cocoa Powder. J. Food Eng. 2017, 199, 42–53. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Ni, D. Effect of Superfine Grinding on Quality and Antioxidant Property of Fine Green Tea Powders. LWT Food Sci. Technol. 2012, 45, 8–12. [Google Scholar] [CrossRef]
- Fang, Y.; Selomulya, C.; Chen, X.D. On Measurement of Food Powder Reconstitution Properties. Dry. Technol. 2008, 26, 3–14. [Google Scholar] [CrossRef]
- Wu, D.; He, Y.; Feng, S.; Sun, D.-W. Study on Infrared Spectroscopy Technique for Fast Measurement of Protein Content in Milk Powder Based on LS-SVM. J. Food Eng. 2008, 84, 124–131. [Google Scholar] [CrossRef]
- Selomulya, C.; Fang, Y. 15—Food Powder Rehydration. In Handbook of Food Powders; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 379–408. ISBN 978-0-85709-513-8. [Google Scholar]
- Saggin, R.; Coupland, J.N. Ultrasonic Monitoring of Powder Dissolution. J. Food Sci. 2002, 67, 1473–1477. [Google Scholar] [CrossRef]
- Forny, L.; Marabi, A.; Palzer, S. Wetting, Disintegration and Dissolution of Agglomerated Water Soluble Powders. Powder Technol. 2011, 206, 72–78. [Google Scholar] [CrossRef]
- Fournaise, T.; Burgain, J.; Perroud, C.; Scher, J.; Gaiani, C.; Petit, J. Impact of Formulation on Reconstitution and Flowability of Spray-Dried Milk Powders. Powder Technol. 2020, 372, 107–116. [Google Scholar] [CrossRef]
- Burgain, J.; Scher, J.; Petit, J.; Francius, G.; Gaiani, C. Links between Particle Surface Hardening and Rehydration Impairment during Micellar Casein Powder Storage. Food Hydrocoll. 2016, 61, 277–285. [Google Scholar] [CrossRef]
- Fournaise, T.; Gaiani, C.; Petit, J. Descriptive Modelling of Food Powders Reconstitution Kinetics Followed by Laser Granulometry. Chem. Eng. Sci. 2022, 252, 117440. [Google Scholar] [CrossRef]
- Gaiani, C. Étude des Mécanismes de Réhydratation des Poudres Laitières: Influence de la Structure et de la Composition des Poudres; Institut National Polytechnique de Lorraine: Vandoeuvres-lès-Nancy, France, 2006. [Google Scholar]
- De Richter, S.K.; Gaudel, N.; Gaiani, C.; Pascot, A.; Ferrari, M.; Jenny, M. Swelling of Couscous Grains under Saturated Conditions. J. Food Eng. 2022, 319, 110910. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.; Zheng, H.; Brennan, C. The Effects of Dairy Ingredients on the Pasting, Textural, Rheological, Freeze-Thaw Properties and Swelling Behaviour of Oat Starch. Food Chem. 2018, 245, 518–524. [Google Scholar] [CrossRef]
- Mitchell, W.R.; Forny, L.; Althaus, T.; Niederreiter, G.; Palzer, S.; Hounslow, M.J.; Salman, A.D. Surface Tension-Driven Effects in the Reconstitution of Food Powders. Chem. Eng. Res. Des. 2019, 146, 464–469. [Google Scholar] [CrossRef]
- Sweijen, T.; Chareyre, B.; Hassanizadeh, S.M.; Karadimitriou, N.K. Grain-Scale Modelling of Swelling Granular Materials; Application to Super Absorbent Polymers. Powder Technol. 2017, 318, 411–422. [Google Scholar] [CrossRef]
- Cuq, B.; Rondet, E.; Abecassis, J. Food Powders Engineering, between Knowhow and Science: Constraints, Stakes and Opportunities. Powder Technol. 2011, 208, 244–251. [Google Scholar] [CrossRef]
- Ghandari, B.; Bansal, N.; Zhang, M. (Eds.) Handbook of Food Powders [Texte Imprimé]: Processes and Properties; Woodhead Publishing Series in Food, Technology and Nutrition; Woodhead Publishing: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA, 2013; ISBN 978-0-85709-513-8. [Google Scholar]
- Kirchberg, S.; Abdin, Y.; Ziegmann, G. Influence of Particle Shape and Size on the Wetting Behavior of Soft Magnetic Micropowders. Powder Technol. 2011, 207, 311–317. [Google Scholar] [CrossRef]
- Schuck, P.; Dolivet, A.; Jeantet, R. Analytical Methods for Food and Dairy Powders, 1st ed.; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-0-470-65598-6. [Google Scholar]
- Nguyen, H.N.G.; Zhao, C.-F.; Millet, O.; Selvadurai, A.P.S. Effects of Surface Roughness on Liquid Bridge Capillarity and Droplet Wetting. Powder Technol. 2021, 378, 487–496. [Google Scholar] [CrossRef]
- Dupas, J.; Girard, V.; Forny, L. Reconstitution Properties of Sucrose and Maltodextrins. Langmuir 2017, 33, 988–995. [Google Scholar] [CrossRef]
- Seerangurayar, T.; Manickavasagan, A.; Al-Ismaili, A.M.; Al-Mulla, Y.A. Effect of Carrier Agents on Physicochemical Properties of Foam-Mat Freeze-Dried Date Powder. Dry. Technol. 2018, 36, 1292–1303. [Google Scholar] [CrossRef]
- Gaudel, N.; Gaiani, C.; Harshe, Y.M.; Kammerhofer, J.; Pouzot, M.; Desobry, S.; Burgain, J. Reconstitution of Fruit Powders: A Process–Structure–Function Approach. J. Food Eng. 2022, 315, 110800. [Google Scholar] [CrossRef]
- Cuissinat, C.; Navard, P. Swelling and Dissolution of Cellulose, Part III: Plant Fibres in Aqueous Systems. Cellulose 2008, 15, 67–74. [Google Scholar] [CrossRef]
- Yang, Q.-Z.; Xiao, Z.-G.; Zhao, Y.; Liu, C.-J.; Xu, Y.; Bai, J.-K. Effect of Extrusion Treatment on the Thermal Stability and Structure of Corn Starch with Different Emulsifiers. Czech J. Food Sci. 2016, 33, 464–473. [Google Scholar] [CrossRef]
- Mitchell, W.R.; Forny, L.; Althaus, T.O.; Niederreiter, G.; Palzer, S.; Hounslow, M.J.; Salman, A.D. Mapping the Rate-Limiting Regimes of Food Powder Reconstitution in a Standard Mixing Vessel. Powder Technol. 2015, 270, 520–527. [Google Scholar] [CrossRef]
- Neves, M.I.L.; Desobry-Banon, S.; Perrone, I.T.; Desobry, S.; Petit, J. Encapsulation of Curcumin in Milk Powders by Spray-Drying: Physicochemistry, Rehydration Properties, and Stability during Storage. Powder Technol. 2019, 345, 601–607. [Google Scholar] [CrossRef]
- Mo, C.; Navarini, L.; Liverani, F.S.; Ellero, M. Modelling Swelling Effects during Coffee Extraction with Smoothed Particle Hydrodynamics. Phys. Fluids 2022, 34, 043104. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Yao, S.; Song, H. Aqueous Solubilization and Extraction of Curcumin Enhanced by Imidazolium, Quaternary Ammonium, and Tropine Ionic Liquids, and Insight of Ionic Liquids-Curcumin Interaction. J. Mol. Liq. 2020, 317, 113906. [Google Scholar] [CrossRef]









| Hibiscus Calyx Drying Method | Parameter | Efficiency |
|---|---|---|
| Sun-drying [4,12] Sun-drying using a heat pump [12,46] |
|
|
| Oven-drying [12,46] |
|
|
| Dehumidified-air-drying [12] |
|
|
| Drying of Solids | Drying of Liquid | Solid Transformation |
|---|---|---|
Sun-drying
| Spray-drying
| Grinding
|
| Drying of Solids | Drying of Liquid | Solid Transformation |
|---|---|---|
Sun-drying
| Spray-drying
| Grinding
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’be, C.U.; Scher, J.; Gaiani, C.; Amani, N.G.; Burgain, J. Impact of Processing and Physicochemical Parameter on Hibiscus sabdariffa Calyxes Biomolecules and Antioxidant Activity: From Powder Production to Reconstitution. Foods 2023, 12, 2984. https://doi.org/10.3390/foods12162984
M’be CU, Scher J, Gaiani C, Amani NG, Burgain J. Impact of Processing and Physicochemical Parameter on Hibiscus sabdariffa Calyxes Biomolecules and Antioxidant Activity: From Powder Production to Reconstitution. Foods. 2023; 12(16):2984. https://doi.org/10.3390/foods12162984
Chicago/Turabian StyleM’be, Cho Urielle, Joël Scher, Claire Gaiani, N’Guessan Georges Amani, and Jennifer Burgain. 2023. "Impact of Processing and Physicochemical Parameter on Hibiscus sabdariffa Calyxes Biomolecules and Antioxidant Activity: From Powder Production to Reconstitution" Foods 12, no. 16: 2984. https://doi.org/10.3390/foods12162984